Unforeseen circumstances and operational variances are an inevitable part of any complex process or regulated industry. Whether it’s a minor departure from a standard operating procedure (SOP), an equipment malfunction, or an unexpected test result, these events – known as deviations – require immediate attention and systematic management. A Deviation Report Template serves as a vital tool in this process, providing a structured framework for documenting, investigating, and resolving these occurrences, ensuring that quality, safety, and compliance standards are maintained.
Effective deviation management is not merely about identifying problems; it’s about understanding their root causes, assessing their impact, and implementing robust corrective and preventive actions (CAPA) to prevent recurrence. Without a standardized approach, the potential for oversight, inconsistency, and incomplete investigations significantly increases, posing risks to product quality, patient safety, and regulatory standing.
The complexity of modern manufacturing, research, and service delivery environments necessitates a rigorous system for tracking any departure from established norms. From pharmaceuticals to food production, aerospace engineering to software development, the integrity of operations hinges on the ability to detect, report, and learn from deviations. This systematic approach transforms potential weaknesses into opportunities for continuous improvement.
By adopting a comprehensive template, organizations can ensure that every critical detail surrounding a deviation is captured consistently. This not only streamlines the reporting process but also facilitates thorough investigations, accurate impact assessments, and the development of effective, data-driven solutions. It empowers teams to act swiftly and decisively, safeguarding operational integrity and fostering a culture of quality.
What is a Deviation Report?
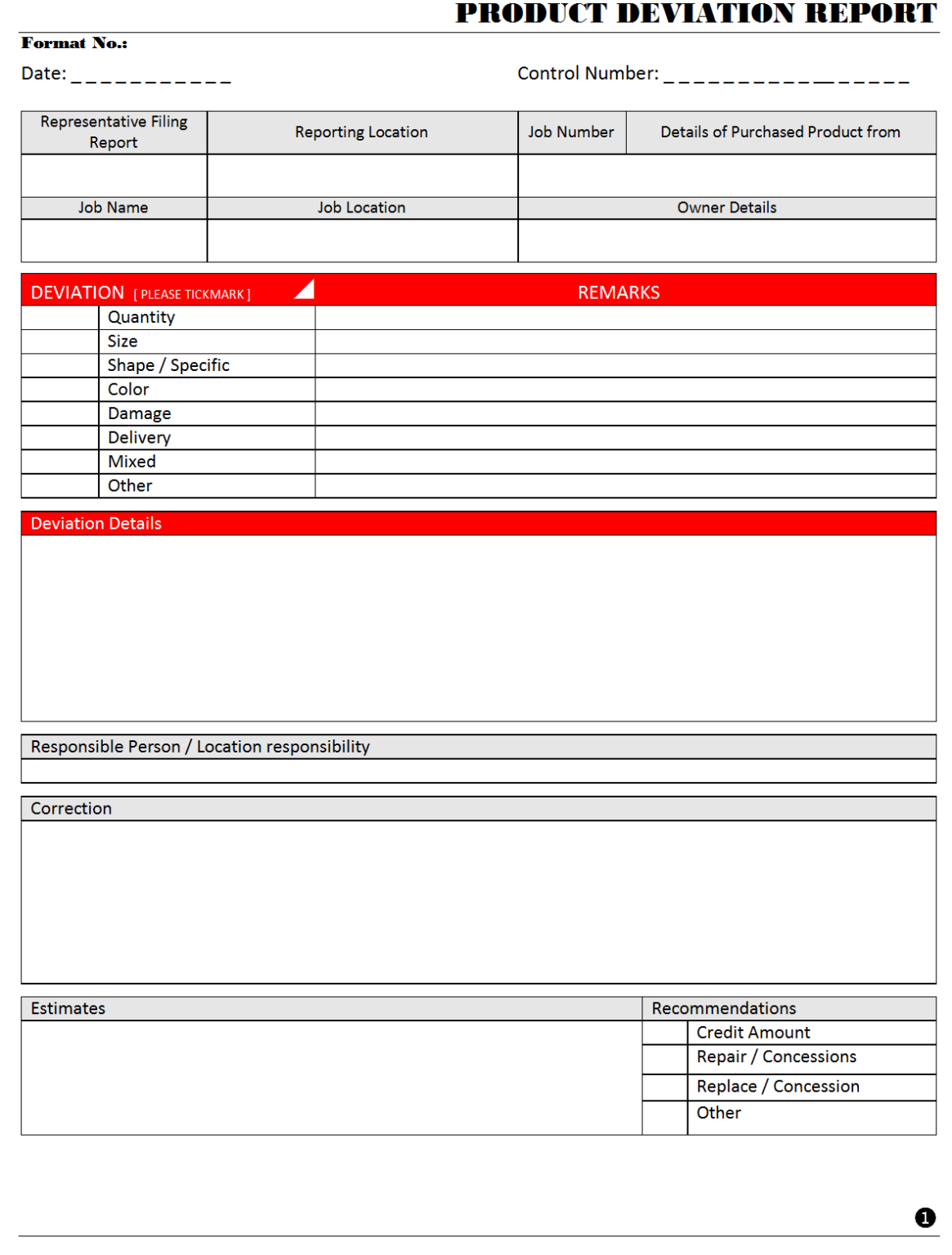
A deviation report is a formal document used to record any departure from approved procedures, specifications, standards, or expected results in a controlled environment. These deviations can range from minor discrepancies, like an out-of-spec environmental reading, to significant incidents, such as a batch contamination or a critical equipment failure. The primary purpose of such a report is to document the event, initiate an investigation into its cause, assess its potential impact, and define appropriate actions to resolve the issue and prevent its recurrence.
In highly regulated sectors, the absence of a robust deviation reporting system can lead to severe consequences, including product recalls, regulatory citations, and damage to reputation. It serves as a cornerstone of a comprehensive quality management system (QMS), providing an audit trail and evidence of due diligence in maintaining compliance with regulations such as Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and ISO standards. Essentially, a deviation report transforms an unexpected event into a structured learning opportunity.
Why is a Deviation Report Template Essential?
Implementing a standardized Deviation Report Template offers numerous benefits, fundamentally improving the efficiency and effectiveness of quality management processes. These advantages span from ensuring compliance to fostering continuous improvement within an organization.
Firstly, a template ensures consistency and standardization across all deviation reports. This means that regardless of who is reporting the deviation, all essential information is captured in a uniform manner, making reports easier to review, compare, and analyze. This consistency is crucial for internal auditing and external regulatory inspections.
Secondly, it promotes thoroughness and completeness. A well-designed template guides the reporter through all necessary fields, prompting them to provide comprehensive details about the deviation, its investigation, and proposed actions. This minimizes the risk of overlooking critical information that could hinder effective resolution or root cause analysis.
Thirdly, templates significantly streamline the reporting process. By providing a ready-made structure, they reduce the time and effort required to draft a report from scratch. This efficiency allows personnel to focus more on the deviation itself and less on the administrative task of documentation.
Finally, a standardized template facilitates data analysis and trend identification. When all deviation data is captured in a consistent format, it becomes much easier to aggregate, categorize, and analyze. This allows organizations to identify recurring problems, pinpoint systemic weaknesses, and make data-driven decisions to implement long-term preventive measures, driving continuous improvement.
Key Components of an Effective Deviation Report Template
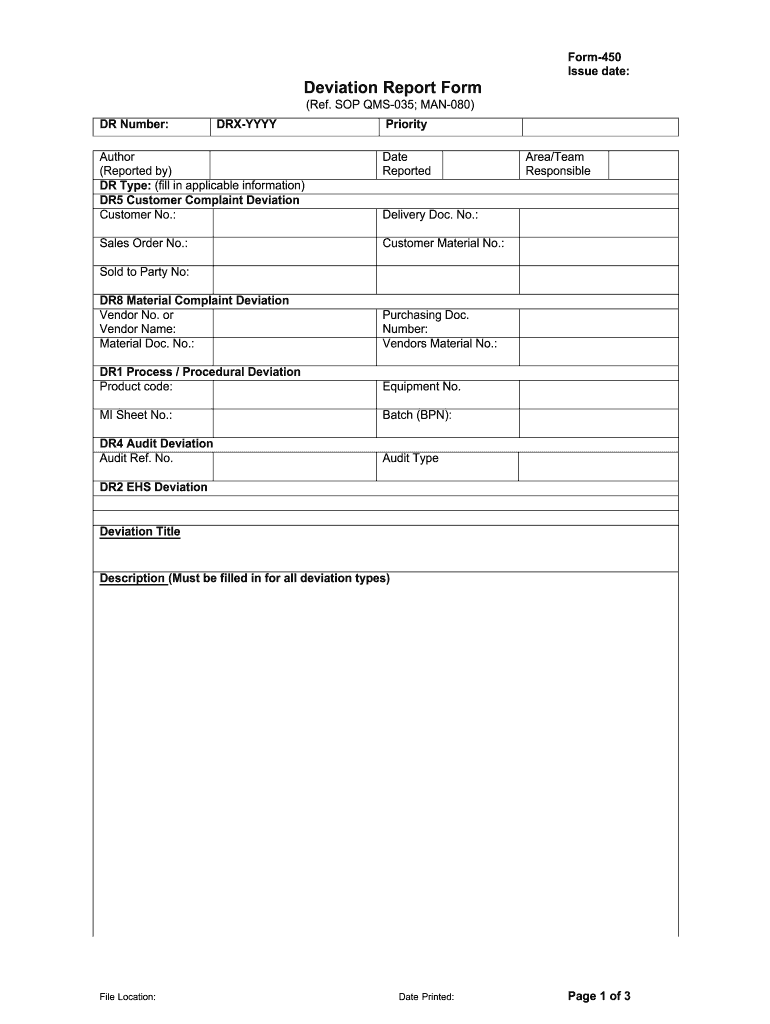
An effective Deviation Report Template is designed to capture all critical information necessary for a thorough investigation, impact assessment, and resolution. While specific fields may vary slightly depending on the industry and the nature of the deviation, the core components generally include:
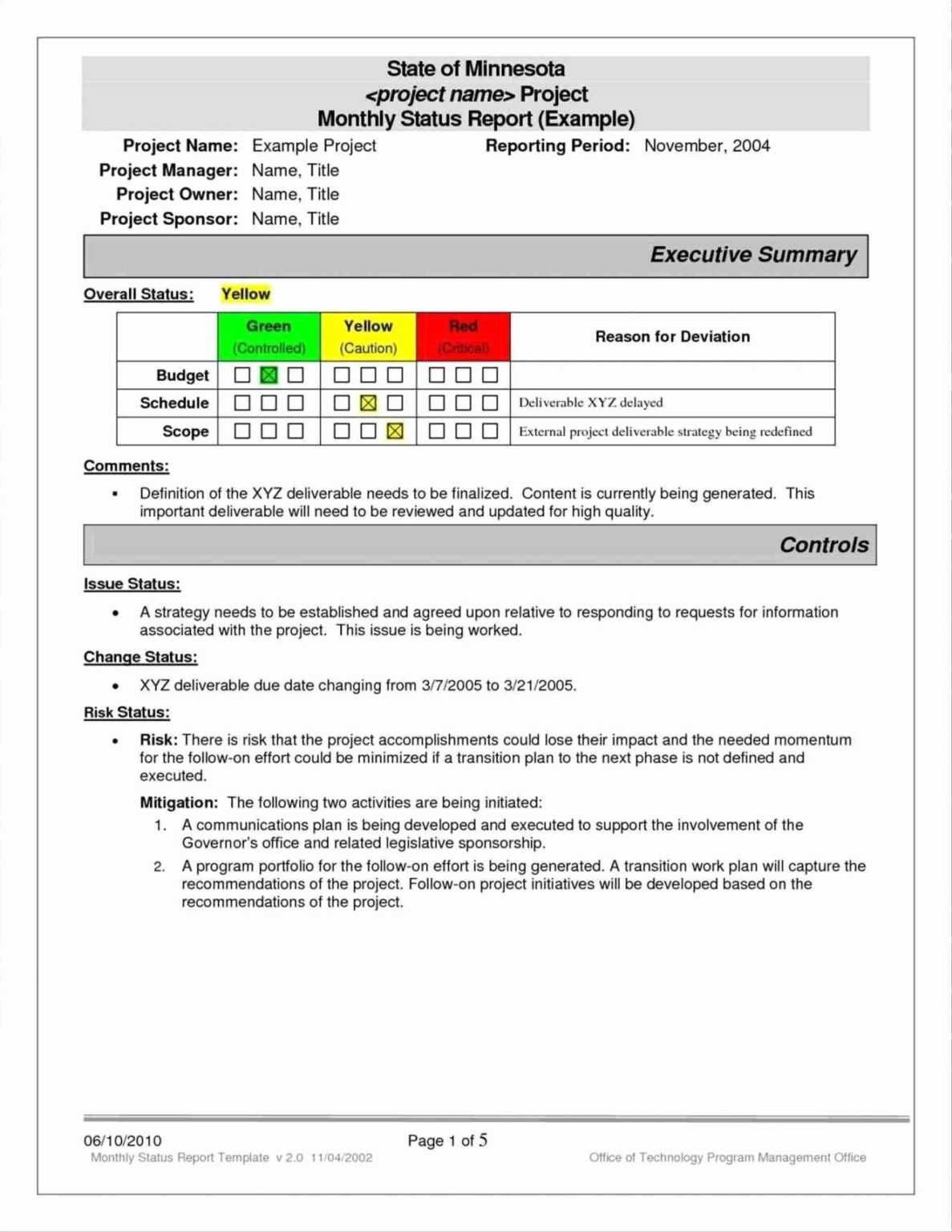
Deviation Report Template Section: General Information
This section provides the administrative details to identify and track the report.
* Deviation Report ID: A unique identification number for easy tracking and referencing.
* Date and Time of Deviation: When the deviation was first observed or occurred.
* Date and Time of Report: When the report was officially documented.
* Reporter’s Name and Department: Who identified and reported the deviation.
* Product/Process/System Involved: Specific details about what was affected (e.g., Batch Number, Equipment ID, SOP Number, Project Name).
* Severity/Risk Level: An initial assessment of the potential impact (e.g., High, Medium, Low) to prioritize investigation.
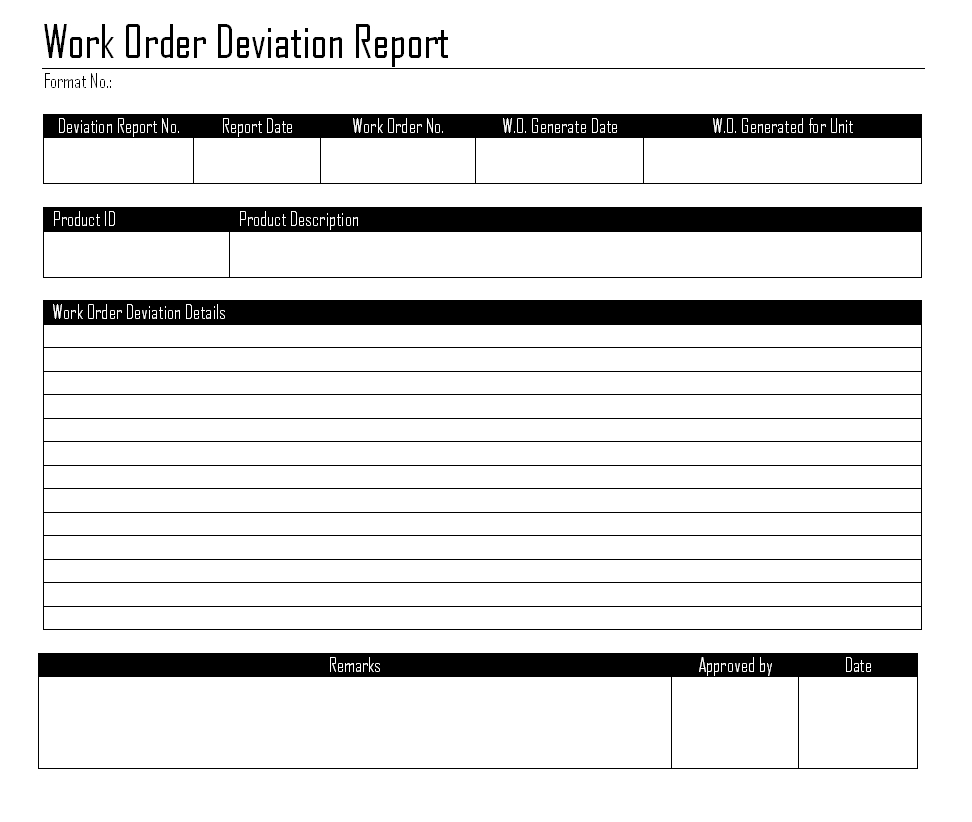
Deviation Report Template Section: Description of Deviation
This is where the factual account of the deviation is documented.
* Detailed Description of the Deviation: A clear, concise, and objective narrative explaining exactly what happened, including specific parameters or conditions that were out of spec. Avoid conjecture; stick to observable facts.
* Location of Deviation: Where the deviation occurred within the facility or process.
* Affected Items/Materials/Personnel: Any specific batches, materials, equipment, or individuals directly involved.
* Reference Documents: Any SOPs, master batch records, specifications, or other documents that were deviated from.
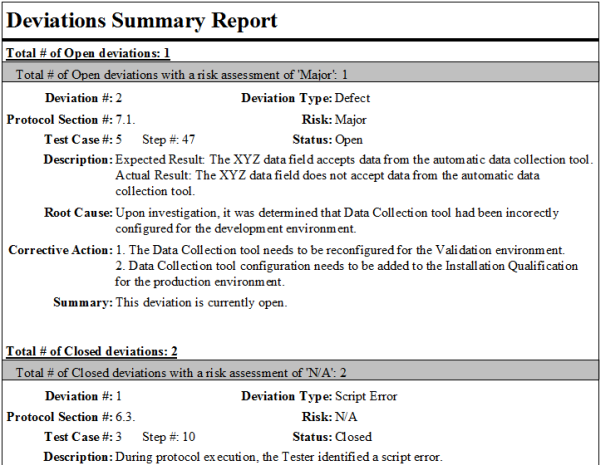
Deviation Report Template Section: Investigation and Root Cause Analysis
This critical section focuses on understanding why the deviation occurred.
* Initial Actions Taken: Immediate steps implemented to contain the deviation or mitigate its impact.
* Investigation Plan: Outline of how the investigation will be conducted.
* Investigation Findings: Detailed summary of facts, observations, and data gathered during the investigation.
* Root Cause Analysis (RCA): The methodology used (e.g., 5 Whys, Fishbone Diagram, FMEA) and the determined root cause(s) of the deviation. This is crucial for developing effective preventive actions.
Deviation Report Template Section: Impact Assessment
This section evaluates the consequences of the deviation.
* Assessment of Impact on Product Quality: Is the product compromised? Does it meet specifications?
* Assessment of Impact on Patient/Consumer Safety: Are there any health or safety risks?
* Assessment of Impact on Regulatory Compliance: Does the deviation violate any regulations or guidelines?
* Assessment of Impact on Schedule/Cost: Any delays, reworks, or financial implications.
* Disposition of Affected Material/Product: What happened to the material/product involved (e.g., quarantining, rejection, rework, release with justification)?
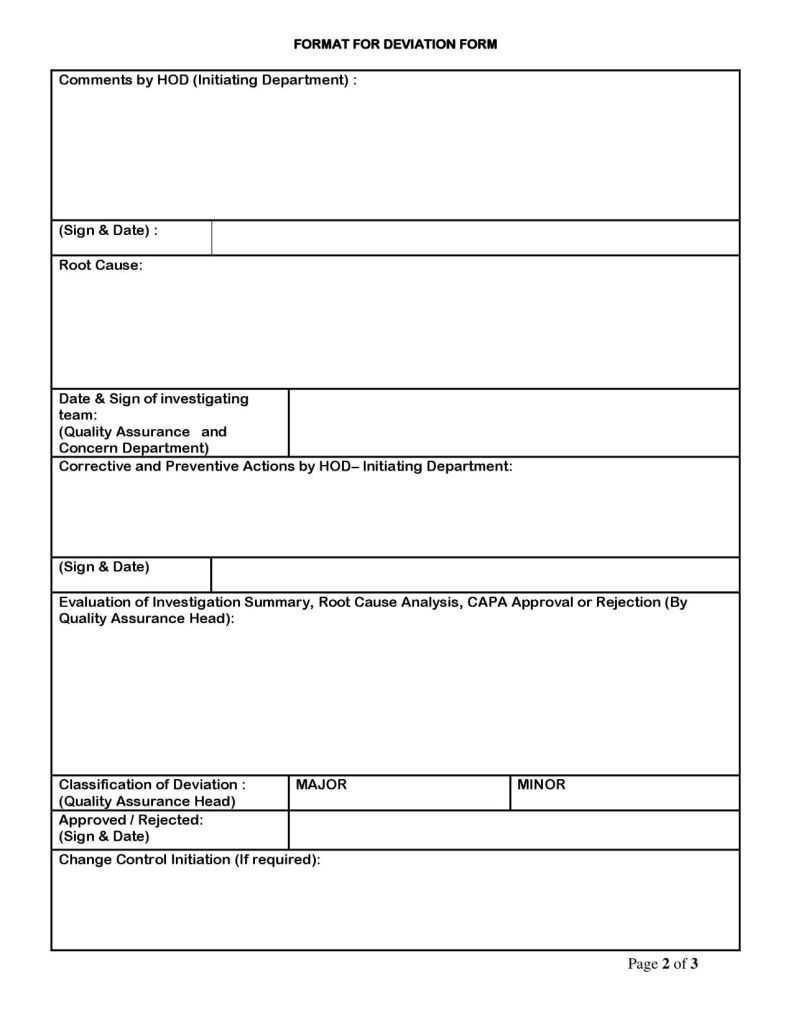
Deviation Report Template Section: Corrective and Preventive Actions (CAPA)
This section outlines the solutions to address the deviation.
* Corrective Actions (CA): Specific actions taken to correct the immediate problem and bring the process back into control.
* Preventive Actions (PA): Actions implemented to eliminate the root cause of the deviation and prevent its recurrence in the future. These should be directly linked to the root cause.
* Responsible Person(s) and Due Dates: Who is accountable for implementing each action and by when.
* Effectiveness Check: How and when the effectiveness of the CAPA will be verified to ensure the deviation does not recur.
Deviation Report Template Section: Review and Approval
The final checks and sign-offs ensuring the deviation has been appropriately managed.
* Reviewer(s) Signatures and Dates: Quality Assurance, Department Head, and other relevant stakeholders.
* Approval Signatures and Dates: Final approval from management or designated authority.
* Closure Date: The date when the deviation report is formally closed, typically after all CAPA have been implemented and verified.
Steps for Completing a Deviation Report
Effectively utilizing a Deviation Report Template involves a systematic process, from initial detection to final closure. Following these steps ensures a comprehensive and compliant approach to deviation management.
- Detection and Initial Assessment: The moment a deviation is observed, it should be immediately documented. The individual detecting it should make an initial assessment of its severity and potential impact.
- Immediate Containment: Take prompt action to contain the deviation, prevent further impact, and secure any affected materials or products. This might involve quarantining a batch, stopping a process, or isolating equipment.
- Initiate the Deviation Report: Fill out the general information section of the Deviation Report Template as accurately and promptly as possible. Include the date, time, reporter’s details, and a preliminary description of what happened.
- Detailed Description: Provide a comprehensive and objective account of the deviation. Answer the “who, what, when, where, and how” questions. Reference any relevant SOPs, batch records, or specifications that were not followed.
- Investigation and Root Cause Analysis: Conduct a thorough investigation to determine the underlying cause(s) of the deviation. Utilize appropriate tools like the 5 Whys or Fishbone diagrams. Document all findings and the identified root cause(s) in the template.
- Impact Assessment: Evaluate the potential impact of the deviation on product quality, patient safety, regulatory compliance, and business operations. Detail any affected materials or products and their disposition.
- Define Corrective and Preventive Actions (CAPA): Based on the root cause, identify specific corrective actions to fix the immediate problem and preventive actions to prevent its recurrence. Assign responsibilities and realistic due dates for each action.
- Review and Approval: The completed report, including the CAPA plan, must be reviewed by relevant stakeholders (e.g., QA, department heads) and approved by authorized personnel. This ensures that the investigation is robust and the proposed actions are appropriate.
- Implementation and Verification of CAPA: Execute the approved corrective and preventive actions. Once implemented, verify their effectiveness through follow-up checks, audits, or re-monitoring of the process.
- Closure: Once all CAPA have been successfully implemented and their effectiveness verified, the deviation report can be formally closed, with all documentation filed for future reference and auditing.
Best Practices for Deviation Reporting and Using Your Deviation Report Template
Maximizing the effectiveness of your Deviation Report Template goes beyond merely filling in fields. Adhering to best practices ensures accuracy, efficiency, and ultimately, a stronger quality system.
- Timeliness is Key: Report deviations as soon as they are identified. Delays can obscure facts, make investigations more difficult, and potentially exacerbate the impact. Prompt reporting demonstrates a proactive approach to quality.
- Be Objective and Factual: Descriptions should be based on observable facts, not assumptions or opinions. Provide clear, concise language and avoid jargon where possible. Attach supporting documentation like photos, log sheets, or instrument printouts.
- Train Your Team: Ensure all relevant personnel are thoroughly trained on how to identify deviations, when to report them, and how to accurately complete the Deviation Report Template. Regular refresher training helps maintain proficiency.
- Link to Your CAPA System: The deviation report should be seamlessly integrated with your Corrective and Preventive Action (CAPA) system. A well-managed deviation often feeds directly into a CAPA to ensure systemic issues are addressed.
- Regular Review and Improvement: Periodically review your deviation reports and the template itself. Are there recurring types of deviations? Is the template capturing all necessary information efficiently? Use this data to refine processes and the template.
- Foster a Culture of Openness: Encourage employees to report deviations without fear of reprimand. Emphasize that reporting is a critical component of quality improvement, not an admission of failure. This promotes transparency and early problem detection.
- Maintain an Audit Trail: Ensure all reports are properly dated, signed, and securely stored. This provides an invaluable audit trail for regulatory inspections and internal reviews, demonstrating a controlled approach to quality incidents.
Industries Benefiting from a Standardized Deviation Report Template
While commonly associated with pharmaceuticals and medical devices, the utility of a standardized Deviation Report Template extends across a broad spectrum of industries where quality, safety, and compliance are paramount.
- Pharmaceutical and Biotechnology: Critical for documenting non-conformances in manufacturing, laboratory testing, and clinical trials to ensure drug safety and efficacy.
- Medical Device Manufacturing: Essential for tracking issues related to product design, production, and post-market surveillance to maintain device reliability and patient safety.
- Food and Beverage: Used to report discrepancies in ingredients, processing, packaging, and hygiene to ensure food safety and compliance with regulations like HACCP.
- Chemical Manufacturing: For documenting deviations in chemical compositions, processes, or safety protocols to prevent hazardous incidents and ensure product integrity.
- Aerospace and Automotive: Critical for reporting manufacturing flaws, design errors, or operational incidents that could impact safety and performance of vehicles and aircraft.
- Electronics Manufacturing: Used to address defects in components, assembly processes, or testing procedures to ensure product quality and reliability.
- Information Technology (IT) and Software Development: While often termed “bug reports” or “incident reports,” these serve a similar function, documenting deviations from expected software behavior or system performance.
- Utilities and Energy: For recording operational anomalies, equipment failures, or safety incidents in power generation, water treatment, or infrastructure management.
In each of these sectors, the structured approach offered by a template not only aids in immediate problem resolution but also contributes significantly to risk management, regulatory compliance, and continuous operational improvement.
Digital vs. Manual Deviation Report Templates
Organizations have choices when it comes to implementing their Deviation Report Template: using traditional manual methods or leveraging digital solutions. Each approach has its merits and drawbacks.
Manual Deviation Report Templates:
Historically, these involve paper forms or basic electronic documents (e.g., Word, Excel) that are filled out manually.
* Pros: Low initial cost, easy to set up, requires minimal technical expertise.
* Cons: Prone to human error, difficult to track and retrieve, challenges with version control, limited analytical capabilities, cumbersome for approvals, not ideal for large volumes. Data aggregation for trend analysis is a time-consuming manual process.
Digital Deviation Report Templates:
These typically reside within specialized Quality Management System (QMS) software, Enterprise Resource Planning (ERP) systems, or dedicated deviation management platforms.
* Pros: Enhanced accuracy and completeness (through validation rules), real-time tracking, automated workflows for approvals, robust data analysis and trend reporting, improved search and retrieval, better security and audit trails, seamless integration with CAPA systems.
* Cons: Higher initial investment (software, training, implementation), requires IT support, potential for resistance to change.
Many organizations start with manual templates and transition to digital solutions as their operations grow and their need for advanced analytics and streamlined processes increases. Hybrid approaches, where reports are initiated manually but then transferred to a digital system, are also common. The choice largely depends on the organization’s size, budget, regulatory requirements, and commitment to digital transformation.
Conclusion
The effective management of deviations is an indispensable element of a robust quality management system in any regulated or process-driven industry. A meticulously designed and consistently utilized Deviation Report Template is far more than a mere administrative form; it is a critical tool that underpins quality assurance, risk mitigation, and continuous improvement. By providing a standardized framework, it ensures that every unexpected event is thoroughly documented, rigorously investigated, and comprehensively resolved. This systematic approach guarantees compliance with regulatory requirements, enhances operational efficiency, and ultimately contributes to the production of high-quality, safe products and services. Embracing a well-structured deviation reporting process, supported by an effective template, empowers organizations to transform challenges into valuable learning opportunities, fostering a culture of excellence and unwavering commitment to quality.
]]>