Navigating the intricate landscape of clinical research demands meticulous data collection, a process critically dependent on well-designed tools. At the heart of this data capture mechanism lies the Case Report Form (CRF), a specialized document or electronic interface used to record all protocol-required information for each study participant. For researchers and study sponsors, the development of these forms can be a time-consuming and complex endeavor, fraught with the potential for errors if not approached systematically. This is precisely where a robust Case Report Form Template becomes an invaluable asset, streamlining the design process and ensuring consistency across studies or within different sites of a multi-center trial.
The importance of accurate and complete data cannot be overstated in clinical trials. It forms the foundation upon which scientific conclusions are drawn, regulatory approvals are sought, and patient safety is assessed. Any inaccuracies or omissions in data collection can compromise the integrity of the entire study, leading to invalidated results, ethical concerns, and significant financial repercussions. Therefore, the design and implementation of CRFs are not merely administrative tasks but critical scientific activities that directly impact the reliability and credibility of research outcomes.
Developing a CRF from scratch for every new study can introduce variability and inefficiencies. Different research teams might inadvertently omit crucial data points, format information inconsistently, or fail to adhere to regulatory standards. Such inconsistencies can complicate data aggregation, analysis, and interpretation, ultimately delaying research progress. The strategic adoption of a standardized template mitigates these risks, providing a proven framework that guides data collection efforts from inception to completion, ensuring every essential data point is considered and captured uniformly.
Furthermore, in an era where regulatory scrutiny is paramount and the volume of clinical data continues to grow, efficiency and compliance are key. A well-constructed template not only simplifies the initial design phase but also facilitates easier review by ethical committees, regulatory bodies, and internal stakeholders. It acts as a blueprint, reflecting best practices and ensuring alignment with international guidelines like ICH Good Clinical Practice (GCP). Embracing such a template is a proactive step towards enhancing data quality, accelerating study timelines, and ultimately bringing new treatments to patients more safely and efficiently.
Understanding the Case Report Form (CRF)
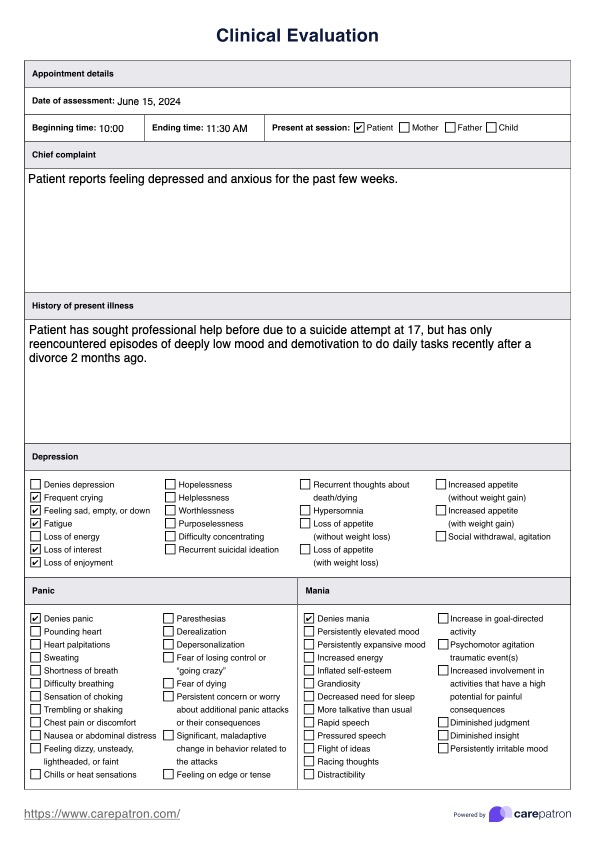
The Case Report Form (CRF) serves as the primary data collection tool in clinical research. It is a structured document, either paper or electronic, designed to capture all the information on each individual study participant as required by the study protocol. This includes demographic details, medical history, physical examination findings, laboratory results, adverse events, concomitant medications, and efficacy endpoints. The meticulous design of a CRF is crucial, as it directly influences the quality, integrity, and reliability of the data collected, which in turn impacts the validity of the study’s conclusions.
What is a CRF?
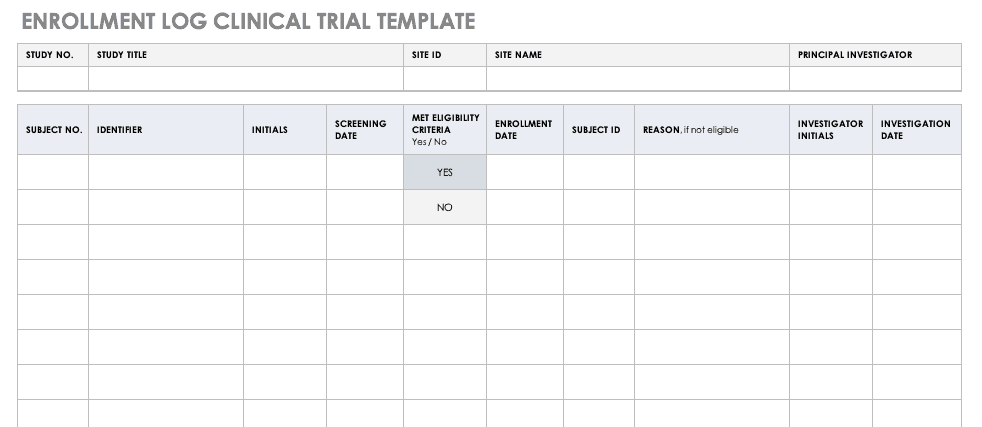
A CRF is essentially a questionnaire, often multi-page, that translates the objectives and data requirements of a clinical trial protocol into a concrete set of questions and fields for data entry. Its purpose extends beyond mere data capture; it ensures that data collection is standardized, complete, and consistent across all participants and study sites. Every piece of information recorded on a CRF must be traceable back to source documents, such as patient medical records, lab reports, or diagnostic images, upholding the principles of data verifiability and auditability.
The Role of CRFs in Clinical Research
CRFs play a pivotal role throughout the entire lifecycle of a clinical trial. During the study design phase, they help translate complex scientific hypotheses into tangible data points. In the operational phase, they guide study personnel in recording patient information accurately and consistently. Post-data collection, CRFs facilitate data entry, cleaning, and analysis, forming the basis for statistical reports and regulatory submissions. They are indispensable for demonstrating drug safety and efficacy, complying with regulatory requirements, and ultimately contributing to scientific knowledge and public health.
The Indispensable Case Report Form Template
The development of high-quality CRFs is a labor-intensive process, often requiring specialized expertise. This is where the concept of a Case Report Form Template provides immense value. A template is a pre-designed, standardized framework for CRFs that can be adapted and customized for specific studies. It incorporates common elements, best practices, and regulatory considerations, significantly reducing the time and effort required to create a new CRF from scratch.
Benefits of Using a Standardized Case Report Form Template
Implementing a standardized Case Report Form Template offers a multitude of benefits, enhancing both efficiency and data quality. Firstly, it ensures consistency in data collection across different sites or studies, which is vital for multi-center trials and meta-analyses. Secondly, it reduces development time and costs by providing a pre-built structure, allowing researchers to focus on study-specific adaptations rather than foundational design. Thirdly, templates inherently improve data quality by incorporating established best practices for clarity, completeness, and adherence to data standards. They often include validation rules, drop-down menus, and clear instructions that minimize data entry errors.
Furthermore, a template can aid in regulatory compliance by reflecting current guidelines and requirements from bodies like the FDA and EMA. This proactive approach helps avoid costly revisions and delays during regulatory submissions. For new research personnel, a standardized template also serves as an excellent training tool, familiarizing them with expected data fields and the overall data collection process. It fosters a culture of accuracy and attention to detail, crucial for the integrity of clinical research.
Types of CRF Templates
CRF templates can broadly be categorized based on their format and specificity. Paper CRF templates are the traditional method, involving printed forms that are manually filled out. While still used in some settings, they are prone to legibility issues, data entry errors during transcription, and slower data processing. Electronic Case Report Form (eCRF) templates, on the other hand, are digital forms accessible via web-based platforms or dedicated software. These offer superior data validation, real-time data access, enhanced security, and faster data processing. Beyond format, templates can also be therapeutic area-specific, designed for oncology, cardiology, neurology, etc., or study phase-specific, tailored for Phase I, II, III, or IV trials, incorporating relevant common data elements for those contexts.
Essential Components of a Robust Case Report Form Template
An effective Case Report Form Template must be comprehensive, covering all necessary data points as dictated by the study protocol and regulatory requirements. While specific content will vary by study, certain core sections are universally vital.
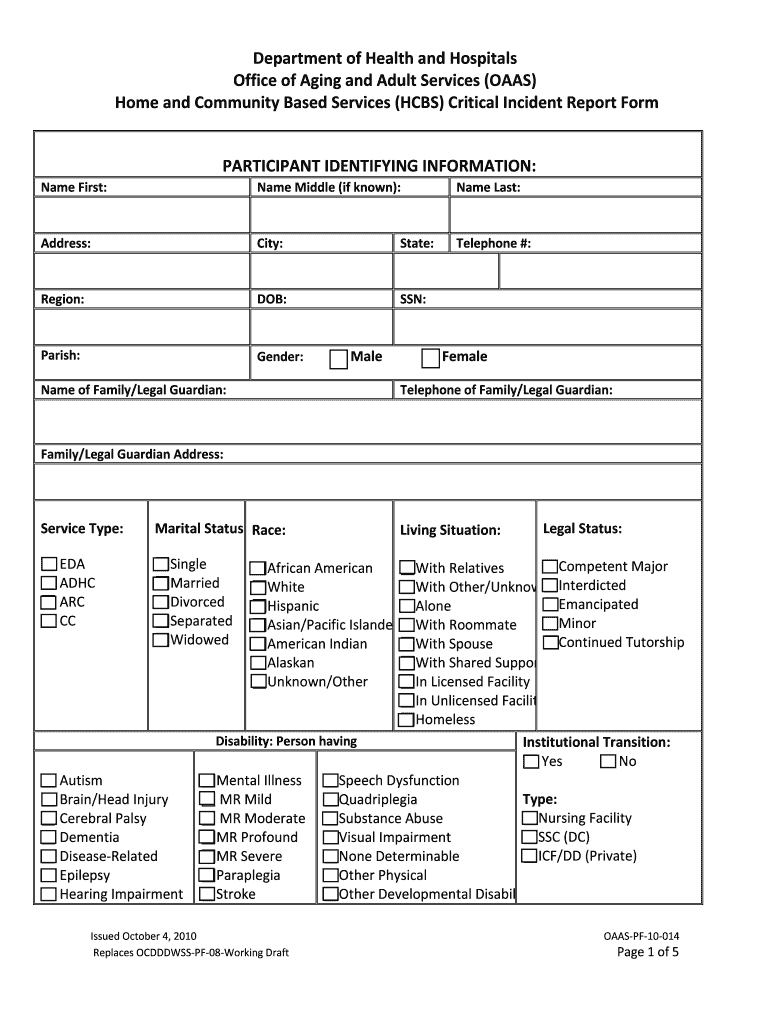
Patient Demographics and Consent
This section typically includes basic patient identifiers (de-identified or coded), date of birth, age, sex, ethnicity, and contact information (if applicable for follow-up, though often managed separately for privacy). Crucially, it also verifies that informed consent was obtained before any study procedures, documenting the date and version of the consent form.
Medical History and Baseline Characteristics
A thorough capture of the patient’s past and present medical conditions, prior surgeries, allergies, and significant family history is essential. This forms the baseline against which any changes or adverse events during the study will be compared. Data points here often include onset dates, diagnoses, and current status of conditions.
Study Procedures and Interventions
This section details all study-specific visits, procedures, and interventions. It documents the administration of the investigational product (dose, route, frequency, date/time), duration of treatment, and any deviations from the protocol. Information on biopsies, imaging, physical examinations, and other assessments performed at each visit is meticulously recorded here.
Efficacy and Safety Data Collection
Efficacy data are the primary and secondary endpoints of the study, measuring the treatment’s effect on the disease. This could include tumor size, symptom scores, lab markers, or quality of life assessments. Safety data focuses on monitoring for adverse events (AEs) and serious adverse events (SAEs), collecting details on their onset, severity, duration, relationship to the study product, and resolution.
Adverse Events and Concomitant Medications
A dedicated section for Adverse Events (AEs) is critical for safety monitoring. It captures detailed information on any untoward medical occurrences experienced by the participant, regardless of causality. This includes the event description, date/time of onset and resolution, severity, seriousness criteria (e.g., hospitalization, life-threatening), outcome, and the investigator’s assessment of causality to the study drug. Similarly, Concomitant Medications records all other medications (prescription, over-the-counter, herbal) taken by the participant during the study, as these can interact with the investigational product or confound results.
Designing an Effective Case Report Form Template
Designing a CRF template requires a blend of clinical knowledge, regulatory understanding, and user interface design principles. The goal is to create a form that is comprehensive yet intuitive, minimizing errors and facilitating efficient data entry.
Best Practices for CRF Design
Effective CRF design emphasizes clarity and conciseness. Each question should be unambiguous, easy to understand, and require a single, direct answer. Use clear instructions, logical flow, and consistent terminology throughout. Employing closed-ended questions (e.g., yes/no, multiple choice, drop-down menus) whenever possible reduces variability and facilitates data analysis. For open-ended questions, provide adequate space and clear guidance on the type of information required. Prioritize the most critical data points and avoid collecting redundant or unnecessary information, which can overburden sites and increase the risk of errors. Implement skip logic for electronic CRFs to hide irrelevant questions based on previous answers, streamlining the user experience.

Customizing Your Case Report Form Template
While a template provides a solid foundation, it must be customized for each specific study. This involves reviewing the study protocol in detail and identifying all unique data points required for the primary and secondary objectives. Researchers should consider the specific disease area, patient population, intervention, and endpoints. Customization may involve adding new sections, modifying existing fields, or adapting validation rules. It is crucial to strike a balance between standardization and study-specific needs. Involving study investigators, statisticians, data managers, and regulatory experts in the customization process ensures all perspectives are considered and the final Case Report Form Template accurately reflects the study’s requirements.
Regulatory and Ethical Considerations
CRF design must strictly adhere to regulatory guidelines, most notably ICH GCP (International Conference on Harmonisation Good Clinical Practice) principles, which mandate that all clinical data be accurately recorded, handled, and stored. CRFs must be designed to capture data in a manner that facilitates source data verification (SDV) and ensures data integrity. This includes clear identification of the study, site, and subject, as well as proper documentation of informed consent. Ethical considerations, particularly patient privacy and data protection (e.g., GDPR, HIPAA), are paramount. CRFs should be designed to collect only the necessary data and should utilize appropriate de-identification or pseudonymization techniques for patient information where possible.
Implementing and Managing Your CRFs
Once designed, the implementation and ongoing management of CRFs are critical to the success of a clinical trial. This involves careful planning, training, and continuous oversight to ensure data quality and integrity.
Data Entry and Validation
For paper CRFs, data is typically manually transcribed into a database, which introduces a risk of transcription errors. This necessitates double data entry or independent verification to minimize mistakes. For eCRFs, data is entered directly into a system, often with built-in validation checks (e.g., range checks, format checks, consistency checks) that flag errors in real-time. This significantly reduces data entry errors and improves data quality at the source. Clear and comprehensive data entry guidelines and training for site personnel are essential, regardless of the CRF format, to ensure consistent and accurate data capture.
Data Management and Quality Control
Effective data management involves a series of processes to ensure the collected data are complete, accurate, and reliable. This includes data cleaning, query generation and resolution (addressing inconsistencies or missing data), coding of medical terms and adverse events, and database lock. Quality control (QC) measures, such as periodic review of CRFs, source data verification (comparing CRF data to original medical records), and internal audits, are critical to maintaining high data quality throughout the study. The chosen Case Report Form Template should facilitate these processes by having a logical structure that makes data review and query resolution straightforward.
The Future of Case Report Forms: eCRFs and Digital Transformation
The clinical research industry is rapidly embracing digital transformation, with electronic Case Report Forms (eCRFs) becoming the standard. This shift offers significant advantages over traditional paper-based methods, paving the way for more efficient and robust data collection.
Transitioning to Electronic Case Report Form Templates
The move from paper to electronic Case Report Form Templates is driven by the need for greater efficiency, accuracy, and real-time data access. eCRFs allow for direct data entry into secure, web-based systems, eliminating the need for manual transcription and reducing associated errors. They can incorporate complex skip logic, automated calculations, and immediate validation checks, improving data quality at the point of entry. Furthermore, eCRFs facilitate real-time monitoring, allowing sponsors and CROs to identify trends, inconsistencies, or potential safety signals much faster than with paper forms. This accelerates decision-making and enhances patient safety.
Leveraging Technology for Data Collection
Beyond basic eCRFs, advanced technologies are further revolutionizing data collection. Integration with Electronic Health Records (EHRs) can automate the transfer of certain patient data, reducing manual effort and potential for errors. The use of wearable devices and mobile applications (ePRO) allows for direct capture of patient-reported outcomes and physiological data in real-time, providing a more comprehensive and ecologically valid picture of a participant’s experience. Artificial intelligence and machine learning are beginning to be explored for automated data review, anomaly detection, and even predictive analytics to identify potential data quality issues before they become significant problems. These technological advancements are continually enhancing the capabilities and efficiency of the Case Report Form Template in modern clinical trials.
Conclusion
The Case Report Form Template stands as an indispensable tool in modern clinical research, serving as the backbone for efficient, accurate, and compliant data collection. From its fundamental role in standardizing information capture to its capacity for significant time and cost savings, the benefits of leveraging a well-designed template are undeniable. It ensures consistency across studies, facilitates regulatory adherence, and ultimately enhances the quality and integrity of the data upon which critical medical advancements depend.
By meticulously designing templates that prioritize clarity, incorporate essential components from demographics to adverse events, and adhere to global regulatory guidelines, researchers can streamline their efforts. The transition to electronic Case Report Form Templates further amplifies these advantages, offering real-time data validation, improved efficiency, and the potential for integration with cutting-edge technologies. As the clinical research landscape continues to evolve, the strategic use and continuous refinement of a robust Case Report Form Template will remain paramount for driving innovation, safeguarding patient welfare, and accelerating the development of new therapies.
]]>